Welcome...
Pharmaceutical Production Suite
Pharmaceutical Production
In 2005, IPEC completed a major turnkey project for an entire Insulin Formulation and Sterile Storage process. Based on the success of that installation, the customer returned to IPEC when they determined that a process expansion was required to increase production capacity. The resulting suite of equipment includes a Formulation Skid, Sterile Storage Skid, two (2) Utility Skids, two (2) Clean-in-Place (CIP) skids, and a Water For Injection (WFI) Cooling skid.

Formulation
The Formulation process consists of fixed and portable vessels for solution preparation in a (ISO 8) clean room. Operations include batching of liquid and dry ingredients, mixing, temperature control, CIP and steam-in-place (SIP) sterilization.

Storage
The Sterile Storage System includes three (3) vessels used for final product storage prior to filling. Both this and the Formulation System are designed for CIP and steam-in-place (SIP).
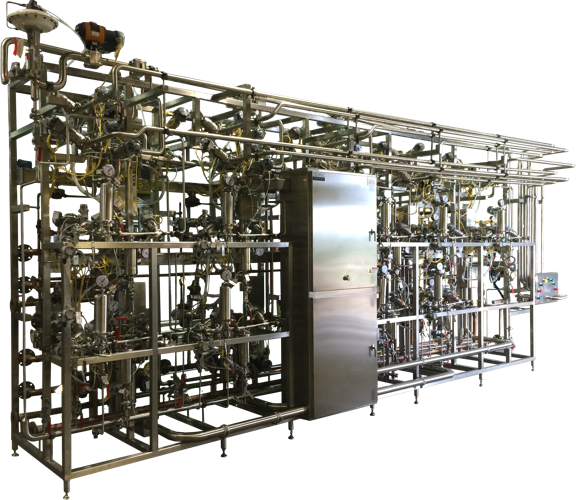
Utility
Each production system includes a dedicated Utility and CIP Module. A common WFI Cooler serves both systems. The Utility Modules route process utilities including clean steam, CIP, process air and chilled water to the process skids.

Featured Projects
CIP
The 2-tank Clean-In-Place Skids prepare and circulate detergent and rinse solutions through the various tanks and piping circuits. Chemical addition equipment and heat exchangers on the CIP Systems control solution concentration and temperature.
Featured Projects
Success
IPEC worked closely with this customer to customize each design for maximum efficiency with minimal hold-up volume, footprint and energy requirements.
This project provides another example of IPEC’s ability to deliver a large order on time to meet a customer’s production schedule. Through the use of modular assemblies we were able to minimize the on-site time required for reassembly and start-up.
This project provides another example of IPEC’s ability to deliver a large order on time to meet a customer’s production schedule. Through the use of modular assemblies we were able to minimize the on-site time required for reassembly and start-up.
